Hey everyone! As a student, you've probably seen or dealt with the Periodic Table. If not, it's one of the most confusing science things ever! My science teacher has worked with my class for about three weeks now, and I know how hard it is to study it. I've collected tips for how the table is organized, what all the letters are for, and all the other things of the Periodic Table of Elements.
First, let's see how this table is organized. In 1869, Dmitri Mendeleev organized the most understandable periodic table. He knew there were more elements to be found, so he left spaces for unknown elements. He organized the elements based on their number of protons and electrons (FYI, the protons and neutrons are what makes up the nucleus, and the electrons are the things around it flying around). If you look at the periodic table, you will see sideways rows, and vertical columns. The rows are called periods, and the columns are families, or groups.
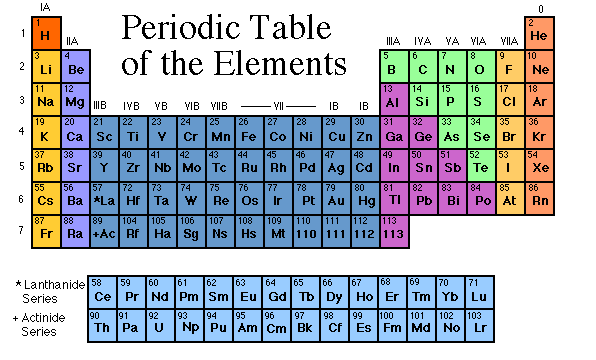
The first group is called the Alkali Metals Family. The only exception is Hydrogen, the very top element. This group contains the most active metal, called Francium. The next group is the Alkali Earth Metals Family. They are slightly less active.
The third through the twelfth groups are called the Transition Metals. These are solid at room temperature.
The thirteenth group is called the Boron Group (Boron is the first element in the family). If you look at some periodic tables, you will see a black zig-zag line, starting in this group. This means that some of these are called metalloids. Metalloids are a mix of metals and non-metals (you'll hear about those later). They are on either side of the line, going down.
The fourteenth group is the Carbon Group. They are both metalloids and metals. The fifteenth and sixteenth groups are the Nitrogen Group and the Oxygen Group. They are just like the Boron and Carbon Groups.
The seventeenth row is special. These are non-metals. They don't react well, and are the complete opposite of metals. They are called Halogens, and include Fluoride and Chlorine. When they mix with Sodium (an alkali metal), they make different kinds of salt. The normal table salt we use is Sodium Chloride, or Sodium and Chlorine.
The last group is called the Noble or Inert Gases. They have full electron spaces, and don't react well. They are the least active group. The only exception is Helium, but is put there because its electron groups are full as well. One familiar Noble Gas is Neon.
I hope this helped with Periodic puzzlings, and that this made it more clear.
I better go study . . .
Logging off (and saying hi to Gabe)
B0BSM1TH